Which Family of Elements Tends to Exist as Monatomic Gases?
ii.5: Some Characteristics of Different Groups
- Page ID
- 86192
Learning Objectives
- Depict how some characteristics of elements chronicle to their positions on the periodic tabular array.
The periodic table is useful for agreement atomic properties that show periodic trends. Periodic trends are specific patterns that are nowadays in the periodic table that illustrate unlike aspects of a certain element, including its size and its electronic properties. Major periodic trends include diminutive radius, melting point, among many other properties every bit we volition talk over. Periodic trends, arising from the arrangement of the periodic tabular array, provide chemists with an invaluable tool to quickly predict an element's properties. These trends exist because of the similar atomic construction of the elements inside their respective grouping families or periods, and because of the periodic nature of the elements.
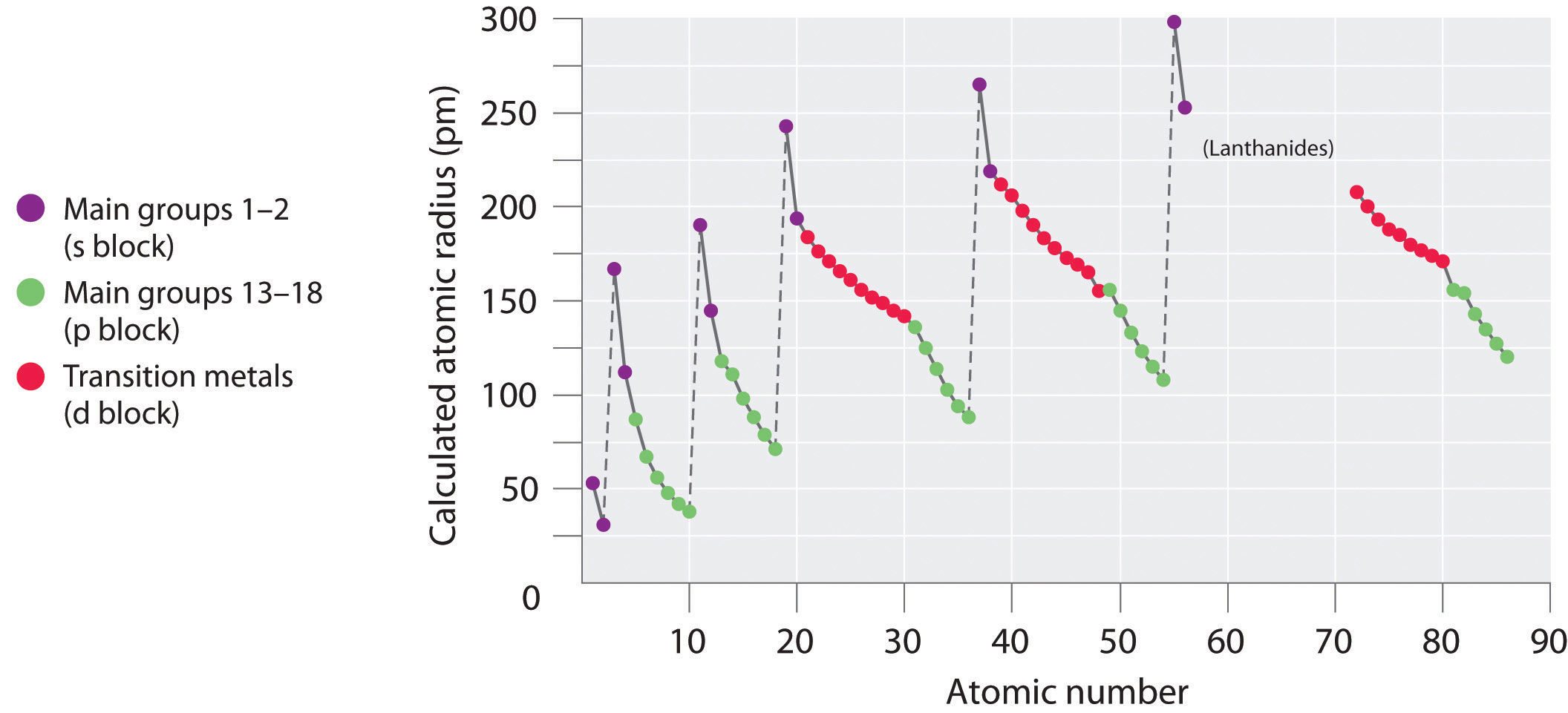
One important atomic belongings is the atomic radius, which is a measure of the atomic size, unremarkably the altitude from the nucleus to the out electron shell. However, since this boundary is non well-divers, at that place are multiple definitions of atomic radius. Irrespective of the definition used, a articulate periodic tendency can be observed when atomic radius is plotted vs. diminutive number (Figure \(\PageIndex{1}\)). The radii generally subtract along each row of the table and increment downward each grouping. The radius increases sharply between the noble gas at the end of each menses and the alkali metal at the beginning of the side by side period. The largest atoms are found in the lower left corner of the periodic table and the smallest are found in the upper right corner
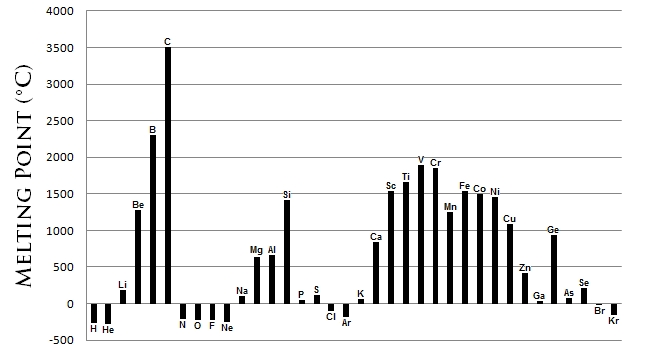
The melting bespeak is a metric of the energy required to transform the solid phase of a substance into a liquid. More often than not, the stronger the bond between the atoms of an element, the more than free energy required to suspension that bail. The melting points exhibit comparable, albeit more than complex, periodic trends as observed in the atomic radii (Figure \(\PageIndex{2}\)). Cardinal feature of these trends are:
- Metals generally possess a high melting indicate .
- Well-nigh non-metals possess depression melting points .
- The non-metallic carbon possesses the highest melting point of all the elements . The semi-metal boron also possesses a high melting point.
The trends of the diminutive radii and melting points (and other chemical and physical properties of the elements) tin be explained by the electron shell theory of the atom discussed in the following sections.
The periodic table is arranged so that elements with like chemical behaviors are in the same group. Chemists oft make full general statements about the backdrop of the elements in a group using descriptive names with historical origins. For case, the elements of Group one are known as the alkali metals, Group 2 are the alkaline metal earth metals, Group 17 are the halogens, and Group 18 are the noble gases.
Group 1: The Alkali Metals
The alkali metals are lithium, sodium, potassium, rubidium, cesium, and francium. Hydrogen is unique in that it is generally placed in Group one, only it is not a metal. The compounds of the alkali metals are mutual in nature and daily life. Ane case is table salt (sodium chloride); lithium compounds are used in greases, in batteries, and as drugs to treat patients who exhibit manic-depressive, or bipolar, behavior. Although lithium, rubidium, and cesium are relatively rare in nature, and francium is so unstable and highly radioactive that it exists in only trace amounts, sodium and potassium are the 7th and eighth well-nigh arable elements in Earth's chaff, respectively.
Group 2: The Alkaline metal Earth Metals
The alkaline earth metals are glucinium, magnesium, calcium, strontium, barium, and radium. Beryllium, strontium, and barium are rare, and radium is unstable and highly radioactive. In contrast, calcium and magnesium are the fifth and sixth nigh arable elements on Earth, respectively; they are found in huge deposits of limestone and other minerals.
Grouping 17: The Halogens
The halogens are fluorine, chlorine, bromine, iodine, and astatine. The name element of group vii is derived from the Greek words for "salt forming," which reflects that all the halogens react readily with metals to form compounds, such every bit sodium chloride and calcium chloride (used in some areas equally route salt).
Compounds that contain the fluoride ion are added to toothpaste and the water supply to prevent dental cavities. Fluorine is also found in Teflon coatings on kitchen utensils. Although chlorofluorocarbon propellants and refrigerants are believed to lead to the depletion of Globe'south ozone layer and comprise both fluorine and chlorine, the latter is responsible for the adverse effect on the ozone layer. Bromine and iodine are less abundant than chlorine, and astatine is and so radioactive that it exists in only negligible amounts in nature.
Grouping xviii: The Noble Gases
The noble gases are helium, neon, argon, krypton, xenon, and radon. Because the noble gases are equanimous of merely single atoms, they are called monatomic. At room temperature and pressure level, they are unreactive gases. Because of their lack of reactivity, for many years they were called inert gases or rare gases. However, the first chemical compounds containing the noble gases were prepared in 1962. Although the noble gases are relatively minor constituents of the atmosphere, natural gas contains substantial amounts of helium. Because of its low reactivity, argon is oftentimes used as an unreactive (inert) atmosphere for welding and in calorie-free bulbs. The red lite emitted by neon in a gas discharge tube is used in neon lights.
To Your Wellness: Radon
Radon is an invisible, odorless element of group 0 that is slowly released from the footing, particularly from rocks and soils whose uranium content is high. Because it is a noble gas, radon is not chemically reactive. Unfortunately, it is radioactive, and increased exposure to it has been correlated with an increased lung cancer gamble.
Because radon comes from the ground, nosotros cannot avoid information technology entirely. Moreover, because it is denser than air, radon tends to accumulate in basements, which if improperly ventilated tin can be hazardous to a building's inhabitants. Fortunately, specialized ventilation minimizes the amount of radon that might collect. Special fan-and-vent systems are available that draw air from beneath the basement floor, before it can enter the living space, and vent it to a higher place the roof of a house.
After smoking, radon is idea to be the second-biggest preventable cause of lung cancer in the United States. The American Cancer Order estimates that ten% of all lung cancers are related to radon exposure. At that place is uncertainty regarding what levels of exposure cause cancer, also every bit what the exact causal agent might exist (either radon or one of its breakdown products, many of which are also radioactive and, unlike radon, not gases). The United states of america Environmental Protection Agency recommends testing every floor beneath the third floor for radon levels to baby-sit against long-term health effects.
Case \(\PageIndex{1}\): Groups
Provide the family/grouping names and menstruation numbers (horizontal values) of each element.
- Li
- Ar
- Ra
Solution:
- Lithium is an brine metal. It is located in period ii.
- Argon is a element of group 0. Information technology is located in period iii.
- Radium is an alkali metal. It is located in menstruum 7.
Example \(\PageIndex{2}\): Classification of Elements
Provide elemental names for the following combinations:
- The brine metal in menstruum three.
- The halogen in menstruation ii
- A metalloid in menstruation four
- A transition metal in period three
Solution:
- Sodium
- Fluorine
- Germanium or Arsenic
- There are no transition metals in menstruation three (gotcha!)
Key Takeaways
- The chemic elements are arranged in a chart called the periodic table.
- Some characteristics of the elements are related to their position on the periodic table.
- The number of valence electrons of an element can be determined by the grouping (vertical cavalcade) number in the Periodic Tabular array. Elements with the aforementioned number of valence electrons have similar chemical properties.
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map:_Fundamentals_of_General_Organic_and_Biological_Chemistry_%28McMurry_et_al.%29/02:_Atoms_and_the_Periodic_Table/2.05:_Some_Characteristics_of_Different_Groups
0 Response to "Which Family of Elements Tends to Exist as Monatomic Gases?"
Post a Comment